• • •
"Mike and Jon, Jon and Mike—I've known them both for years, and, clearly, one of them is very funny. As for the other: truly one of the great hangers-on of our time."—Steve Bodow, head writer, The Daily Show
•
"Who can really judge what's funny? If humor is a subjective medium, then can there be something that is really and truly hilarious? Me. This book."—Daniel Handler, author, Adverbs, and personal representative of Lemony Snicket
•
"The good news: I thought Our Kampf was consistently hilarious. The bad news: I’m the guy who wrote Monkeybone."—Sam Hamm, screenwriter, Batman, Batman Returns, and Homecoming
March 21, 2011
Oh Dear Lord, Help Us All
By: Aaron Datesman
Ordinarily, it would be beneath me to comment on an article (“Damaged Nuclear Power Plants Could Spew Range of Emissions”, March 14, 2011) from the Wall Street Journal. But I read this by accident, and would like to use it to make an unfortunate point.
In a total meltdown, several radioactive gases are released on the less-toxic end of the spectrum, including nitrogen-16, tritium and krypton. Each of these gases is light and tends to dissipate quickly, posing little danger to humans, said Kirby Kemper, a nuclear physicist at Florida State University.Nitrogen-16 decays quickly and becomes stable oxygen. Krypton gas also is very light and would get released into the atmosphere and dissipate quickly.
Kirby Kemper is a Fellow of the American Physical Society, which is a big deal, and the Vice President for Research at FSU. I am not smart enough to ever become either of those things. Do I really need to say that, apparently, Kirby Kemper, Ph.D., is also a very, very big moron?
The molecular weight of krypton is around 84 g/mol; the molecular weight of air, which is mostly nitrogen, is around 28 g/mol. It is not very light by any justifiable standard, and the statement that it will “dissipate quickly” perhaps is only true if you open a cylinder of it while standing on the peaked roof of your house.
It’s possible, of course, that I’m being unfair to Prof. Kemper (who has 262 professional publications on his CV). Maybe the reporter just got it wrong – in which case, it’s a nice touch to get it wrong twice. It is terrifying enough, I think, that our news media is so incompetent regarding an issue which quite literally straddles the line between living and dying for many or all of us.
However, it’s also possible that, after a successful career spent in the field of nuclear engineering, certain opinions become established as fact by way of repetition and groupthink which simply ARE. NOT. SO. (I wrote about this topic here.) For instance, while I have been aware for many years that radioactive noble gases are released by operating nuclear reactors and by reprocessing of nuclear fuel, until I began to dig into this topic last week it literally never occurred to me that krypton and neon are not, in fact, buoyant in air. This is a significant act of brainwashing, I think.
For this reason, I’m afraid that the situation is even more dire than it might appear to be. The media, of course, are totally worthless on the topic of the dangers of radiation and radioactive fallout, as they are useless on almost any substantial topic. This is bad enough on its own. However, the experts who do exist, the ranks of scientific experts in nuclear technology and nuclear engineering, in many cases appear to have been captured by the relentless brainwashing about the safety of nuclear technology that our society has endured for decades.
It would seem that we can’t count on technical experts to apply commonsense knowledge they learned in high school to the industry about which we trust them to advise us. Oh Dear Lord Help Us All.
UPDATE: Thanks to a reader for pointing out an error in this post. Neon is, in fact, lighter than air. I meant, "krypton and xenon", both of which are noble gases, heavier than air, and fission products. Sorry about that.
For the physics-inclined, it turns out that it's not hard to test the assertion made in this post against some numbers. The proper framework is given by the Chapman-Enskog Theory, which is derived from the Boltzmann transport equations.
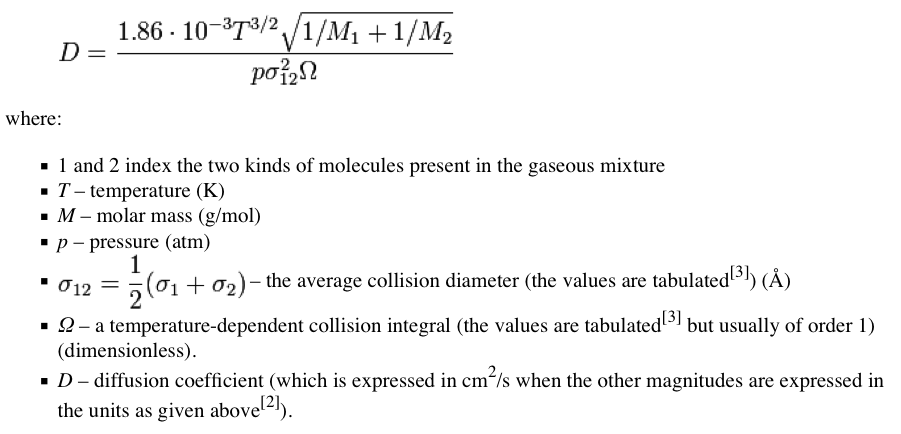
For carbon dioxide (CO2) diffusing in air, the diffusivity coefficient D=16 mm^2/sec. I don't have access to a table which would let me calculate the value exactly, but examining the formula it's clear that the diffusivity of Kr in air can not be much different than that of carbon dioxide. It follows from this that the characteristic diffusion length over a one hour time span is 480 mm - less than two feet. This is basic undergraduate physics.
It's a bit complicated to explain exactly what the characteristic diffusion length is, but it's basically this: take a huge weather balloon on smooth, flat ground, filled with krypton. At time t=-20 seconds, I dissolve the balloon in acid. (This is chosen as the time origin since there is an abrupt discontinuity when the balloon disappears.) At t=0 seconds, I will obviously have a big, round cloud of krypton which has started to blur around its edges, so it's a bit bigger than the balloon.
After one hour, in the absence of wind, that cloud of krypton will be about 960mm larger in diameter. I would not characterize that cloud as dispersing quickly.
— Aaron Datesman
The physics of radioactive gases is more complicated than that, I'm afraid. "Heavy" gases don't pool along the ground like water in oil; they do sink to some extent, but they also diffuse in all directions (especially outdoors where they're pushed and pulled by the wind). Even large clouds of toxic gas will dissipate in a matter of minutes (see eg. World War I mustard gas). That's a problem when that gas can kill you in seconds, but radioactive gases generally take hours to give a dangerous dose. The reason for that is that radioactivity is a mass phenomenon, and even a thick cloud of radioactive gas is not very dense.
Radioactive dust is a more serious problem, but unlike Chernobyl which burned smokily for months, the Fukushima plant has released hardly any dust.
Posted by: AndrewC at March 21, 2011 08:01 PMAndrewC,
Go find a big hole, like the foundation for a house which hasn't been finished yet. Take a bottle of krypton with you. Open the valve while standing in that big hole.
I admit to never having done the experiment, but I'd be shocked if there isn't still a lot of krypton there hours later - quite possibly days later, if it's not windy.
Look, consider the reverse: why is there no helium in the atmosphere? Light molecules rise (and escape). Heavy molecules fall. On average.
You're overthinking.
Posted by: Aaron Datesman at March 21, 2011 08:18 PMIt looks to me to be pretty common sensical that light gases dissipate much more quickly than heavy gases because a heavy gas "tends to sink" and so "will travel further along the ground." But somebody authoritative says it here:
http://www.chemicalspill.org/OffSite/modeling.html
I don't doubt that high winds would still get rid of the heaviest gas as fast as Dorothy's house in the Wizard of Oz, but absent wind the heavy gas would tend to pool, and if confined by topography, as in a valley, would form a fog. As long as we keep the physics at that level, I'm good, so I think nuclear physicists can probably grasp that if properly motivated.
There will be no overthinking from me on this topic.
Posted by: N E at March 21, 2011 08:51 PMI have to admit, I do not understand much of the discussion that has been going on in the last few posts. I DO KNOW however that exposure to radio activity is certainly detrimental to one's health.
Something I definitely understand is below......
http://www.dailykos.com/story/2011/03/20/958417/-SoCal-Windspill-Sparks-Huge-Solar-Radiation-Leaks
h/t Assaf Oron
Posted by: Rupa Shah at March 21, 2011 09:22 PMOh, and please do not miss all the comments that follow.
Posted by: Rupa Shah at March 21, 2011 09:26 PMAs a pilot, I'm familiar with the forces of weather and terrain constantly stirring the air, both horizontally and vertically. If air were stagnant to the extent that a cloud you saw at sunrise was still there at sunset, then likely gasses would separate by molecular weight. Compare a muddy river to a lake.
Posted by: Henry Bayer at March 22, 2011 12:53 AMAaron
Heavy molecules fall. On average.
Hoping to avoid the issue of who's overthinking and who's underthinking, I submit that AndrewC's point is not trivial. You'll note that atmospheric gases are not stratified according to molecular weight. Otherwise (neglecting the trace components), we'd be living in a layer of pure O2, beneath a layer of pure N2.
On short time scales, in the presence of horizontal barriers, dense gases will pool below and lighter gases will be trapped above. But diffusion, convection, and winds are powerful mixing mechanisms, and all are at play in the TMI and Fukushima incidents.
P.S. What about the ozone in TMI?
P.P.S It's a jungle out there amongst the "physics types".
Posted by: SunMesa at March 22, 2011 01:57 AMSunMesa -
Yes, the point is not totally trivial. To be technically correct about it, I suppose I would say that in general the atmosphere is both convectively and diffusively mixed. (Perhaps there is an atmospheric scientist reading who can comment more fully.) In the absence of convective mixing (wind), a pool of dense gas will still dissipate via diffusion. The time constant for that process depends upon physical parameters including the molecular weight.
I see no reason to think that a large quantity of krypton gas in a depression would dissipate in minutes by diffusion. I think we agree on this point.
The Kemper comment (if he said it - he might have been misquoted) is clearly wrong. AndrewC's comment irks me because it sounds like "the physics of radioactive gases" is a complicated field that only nuclear physicists should declaim on. It's not. The behavior of dense gas in atmosphere is the same whether the constituent molecules are radioactive or not.
Let me tell you what convinces me that I am right on this point. Have you ever been to Monticello? It's on a hill without much access to water, which seems like an odd decision for old TJ to have made. But he placed his home there because he knew something interesting. Cold air sinks, displacing warm air.
When there was frost in the valleys around Monticello, Jefferson's fields and orchards were free of frost because they were covered by the warm air displaced from the valleys. The mass difference between cold air and warm is much less than the mass difference between krypton and air.
I don't debate that, even in the absence of convection and wind (aren't those two things the same?), a pool of dense gas will diffuse away eventually. This is a basic result from statistical mechanics - freshman physics, even. But it will not happen in minutes if the gas is pooled in a depression in the absence of wind.
A Fellow of the American Physical Society ought not to be relying on the assumption that the earth is perfectly smooth and flat.
Posted by: Aaron Datesman at March 22, 2011 05:09 AMSunMesa, also, I have the last Holowka post set in my mind. Thanks for asking about it. Your comments and others have been valuable to me in getting my head around it. I'll write it up and post it in a few days.
However, I feel a bit ghoulish writing about her story. It is not a fictional account from a mystery thriller. I didn't know the woman or her family. The series of posts I intended to write center around more personal concerns, and I will be moving back in that direction. That said, I will finish the discussion.
I don't mean to be mysterious. It's reasonably clear when you think about it. If a cloud of radioactive gas did settle on the Holowka farm, I have argued that she would have seen effects resulting from ionization of the atmosphere. Principally, I discussed how the blue glow would have been the result of the ionization of nitrogen.
This leaves around 25% of the atmosphere out of the discussion. Ozone would also be produced from the ionization of molecular oxygen. One would also expect to find dissociation products including CO, NO, and NO2. These gases are very dangerous, and there could well have been high concentrations of them.
Posted by: Aaron Datesman at March 22, 2011 05:28 AMActually Kirby Kemper happens to be right.
Smoke, for example, consists mainly of particles which are heavier than air. Often it consists of small particles of solids and liquids.
It rises because of heat, and may not settle even in a closed jar because of microscopic motion of the air.
Krypton gas emitted by a reactor would be like an invisible smoke.
Posted by: Mark S at March 22, 2011 06:27 AMMark S - where do those particles go?
Blow smoke into a jar and seal it for a week. Wipe the inside of the jar with a paper towel.
Posted by: Aaron Datesman at March 22, 2011 06:30 AMSmoke particles just drift around and, yes, tend to settle on surfaces eventually.
But when dispersed in the whole atmosphere the krypton would be spread so thinly that the only effect would be to raise the background radiation by an infinitesimally small amount, probably too small even to measure, except perhaps in the immediate vicinity.
Posted by: Mark S at March 22, 2011 06:53 AMMark S -
Yes, but HOW LONG will it take for the Kr gas to diffuse to an isotropic abundance? It makes a difference if you're sitting in a cloud of it and it's radioactive.
The answer is not minutes and, in certain terrain at least, not hours. In Marie Holowka's case, it seems to have been days. An atom of Kr has a mass three times as large as a typical molecule of air. It will not diffuse very rapidly in air. This is very basic physics.
That a big-shot scientist like Kemper overlooks complications like this one is symptomatic of a systematic failing of the scientific establishment. Don't dismiss it.
Posted by: Aaron Datesman at March 22, 2011 09:30 AMI find this all very interesting, so thanks you denizens of the physics jungle. As I understand the issue, the real question is whether diffusion, convection, and winds would ALWAYS result in the dispersion of radioactive gases and prevent them from EVER, under any possible atmospheric conditions, causing the physical effects--intense blue glow, sense of heat, pronounced lack of oxygen--the Holowkas described without dying within a matter of days. It's one thing if it could never have happened because, as Donald Johnson initially posited, there just would have had to have been way too much gamma radiation so people would have quickly died from radiation sickness. But it's another thing altogether if Aaron's theory hinges on whether a cloud of radioactive gas could last a while before dissipating or being blown away. The atmospheric conditions necessary to permit that to have happened need not be any more common than an enormous earthquake and tsunamai. Unlikely events happen all the time, which is obviously good for the designers and neighbors of nuclear plants to remember. And maybe it would be good for the rest of us to remember too, if we can still manage to cross the street, drive a car, take medicines, eat food cooked by others, etc. That last bit is I think a big part of why people with authority so consistently feel it's necessary to lie to us all the time--"the public" will never be able to assess risks reasonably and without panic.
Posted by: N E at March 22, 2011 09:44 AMeat food cooked by others
It's definitely worth the trouble to avoid foods that can be problematic if they are stored lukewarm for long periods of time
the paradigmatic example, used in Epi 101 - the egg salad
my own worst food poisoning episode - chili at Wendy's (back when they still had the suggestive corporate motto - pertaining to their hamburgers, of course - "hot and juicy")
Posted by: mistah charley, ph.d. at March 22, 2011 11:44 AMmistah charley
I remember decades ago wolfing down a hamburger with lettuce in Bogota and then, after it was half gone, watching the guy who was with me carefully remove the lettuce from his because, so he said, he personally didn't like amoebas.
It was a great burger, and the amoebas didn't get me. I have on occasion wondered if he was just messin' with me.
Posted by: N E at March 22, 2011 01:13 PM> it literally never occurred to me that krypton and neon are not, in fact, buoyant in air
The atomic weight of Neon is about 20. Sounds buoyant to me.
Good eye there Brugle. Neon looks a little buoyant to me, too, unless that means something more than I would think. And now Brugle, thanks to you indirectly and Wikipedia directly, I also know that neon, though rare on earth, is the fifth most abundant element in the universe. (The fact that this is known somehow by someone baffles and impresses me.) I now have a new vision of the cosmos lit up like Las Vegas.
Posted by: N E at March 22, 2011 06:32 PMAaron (to AndrewC):
Go find a big hole, like the foundation for a house which hasn't been finished yet. Take a bottle of krypton with you. Open the valve while standing in that big hole.
I admit to never having done the experiment, but I'd be shocked if there isn't still a lot of krypton there hours later - quite possibly days later, if it's not windy.
(later, to me):
I see no reason to think that a large quantity of krypton gas in a depression would dissipate in minutes by diffusion. I think we agree on this point.
I would have to agree on an intuitive basis, but why speculate? Let us be bold, a rough calculation of the diffusion timescale ought to be tractable. [At least for nerds, which probably includes most of the readers still following this thread... bless your hearts.]
I don't have data for the diffusion coefficient of krypton into air, but it can be approximated by assuming that the diffusion coefficient of a gas is proportional to the square-root of its density (Graham's Law). Using the known self-diffusion coefficient for N2 (0.221 sq.cm/gm), the corresponding value for krypton into air is ~0.127 sq.cm/gm. Assume further (as is often done) that this value is constant throughout the process.
At this point it's simply a matter of solving the equation for 1-D diffusion from a planar 'slab' of gas, with a rigid barrier on one face (the bottom of the pit), and a semi-infinite volume of air on the other. I set the height of the slab to be 8 ft.
There is no closed-form solution to this problem, but it is straightforward to obtain by numerical integration. Assuming I formulated/coded this correctly (probably the shakiest assumption of the lot), the time required for the mean concentration of krypton in the pit to drop by a factor of 2 is somewhere between ~5 and ~120 hrs.
Why this broad range? Well, the higher value represents the literal 1-D solution, which neglects the horizontal diffusion from the sides of the gas volume above the pit, and so overestimates the actual time required. The lower value comes from keeping the initial (high) rate of diffusion unchanged throughout the process, which corresponds to the gas above the pit being continuously replaced with fresh air. This would underestimate the actual diffusion time, although it may reasonably capture the effect of a mild breeze. A stiff wind would obviously reduce this value much further, but that is a more difficult problem to model.
So, bottom line is, under strictly static atmospheric conditions, an 8 ft deep pit of krypton gas could indeed retain a substantial fraction of its initial concentration for several days. Under more realistic (but not severe) conditions, the timescale is probably much shorter, perhaps only several hours. All consistent with your hunch above.
...even in the absence of convection and wind (aren't those two things the same?)
Technically, convection refers to mass transport driven by differences in density (in the presence of gravity), whereas wind derives from differences in pressure.
Posted by: SunMesa at March 22, 2011 08:15 PMHmm SunMesa....it seems we were working on the same problem at the same time.....
Posted by: Aaron Datesman at March 22, 2011 08:27 PMGassified Cobalt prehaps???
Posted by: Mike Meyer at March 22, 2011 08:50 PMI haven't studied this much, but my impression was that molecular diffusion isn't all that relevant in spreading stuff around for the very reason you point out. Turbulent diffusion does most of the work. If someone lights a cigarette you don't have to wait a few hours to smell it. Now of course there's convection taking place there, but if you put the smoke inside a jar, let it reach room temperature and then opened the jar, would it really take hours for the smell to reach your nose a few feet away? My guess is no. I assume the molecular weight of the smelly stuff in tobacco smoke is fairly high, but don't know.
Or if someone steps in an elevator wearing a lot of perfume, in my experience you don't need to wait an hour to notice.
My extremely limited knowledge of this stuff comes from mixing length theory, where the diffusion coefficient is some characteristic velocity times a characteristic length. So the diffusion coefficient depends on the size of the random little eddies times their velocity--is there an atmospheric physicist who could say what that's likely to be on a calm day?
I'm not sure this is relevant to whether TMI killed anyone, but the physics is interesting. The relevant post is the one following,with the mortality figures, and not having any competence in that area I'll just lurk.
Posted by: Donald Johnson at March 22, 2011 10:14 PMDonald Johnson -
I'm not an expert at this, either. But my opinion is that our intuition about how heavy gases in atmosphere isn't well developed. I can never recall doing a problem on this topic. So the math is all I have to go by.
Well, that, and the Monticello example. Which I find to be very powerful, actually.
Posted by: Aaron Datesman at March 22, 2011 10:29 PM"I went to the barn around four, four-thirty [in the morning]. We were milking cows. And the barn started to shake. And I heard a rumble like underground. Well, I wouldn’t say an earthquake. But it was going like 'brrup, brrup, brrup'. And then it shook and shook and we didn’t hear the big rumbles. But every now and then you could hear a rumbling in the ground. And Paul, my brother, was with me and he says, 'That’s an earthquake.' I said, 'Paul, it don’t sound like earthquake. Earthquake, it just rattles. But you don’t hear the noise, the brrup, brrup.' It just [was] like there was boiling water coming underground. And I said, 'l think something happened at Three Mile Island.' Then we kept on milking.
And Paul left me about six o’clock." . . .
"[It is quite certain that Marie’s above account took place on March 28th. However, her account of media coverage on evacuation follows right after, leading to the possibility that this passage may have occurred on Thursday."
-Marie Holowka, Zion’s View
Farmer
Interviewed: August 12, 1986
http://www.tmia.com/node/118
Zion's View is roughly seven miles south of the island and there are about three towns between the two locations. If the sound she heard was coming from TMI then those closer would have heard it much better than she did. Where did that sound come from. If it was, as she described it, "like there was boiling water coming underground," then that noise would be a cavity of gas in a liquid.
I don't know the plumbing of a reactor so I don't know if it's even possible for the pressure to back feed a well but they did have a hydrogen explosion that blew doors off hinges and claimed they did not leak into the atmosphere.
"In 2005 R. William Field, an epidemiologist at the University of Iowa, who first described radioactive contamination of the wild food chain from the accident suggested that some of the increased cancer rates noted around TMI were related to the area's very high levels of natural radon, noting that according to a 1994 EPA study, the Pennsylvania counties around TMI have the highest regional screening radon concentrations in the 38 states surveyed."
-http://en.wikipedia.org/wiki/Three_Mile_Island_accident_health_effects#cite_ref-4
From my very shallow understanding gleaned from several days at wikipedia, radon is a granddaughter product of uranium.
"A natural nuclear fission reactor is a uranium deposit where analysis of isotope ratios has shown that self-sustaining nuclear chain reactions have occurred. The existence of this phenomenon was discovered in 1972 at Oklo in Gabon, Africa, by French physicist Francis Perrin."
. . .
"The natural nuclear reactor formed when a uranium-rich mineral deposit became inundated with groundwater that acted as a neutron moderator, and a nuclear chain reaction took place. The heat generated from the nuclear fission caused the groundwater to boil away, which slowed or stopped the reaction. After cooling of the mineral deposit, short-lived fission product poisons decayed, the water returned and the reaction started again. These fission reactions were sustained for hundreds of thousands of years, until a chain reaction could no longer be supported."
. . .
"A key factor that made the reaction possible was that, at the time the reactor went critical, the fissile isotope 235U made up about 3% of the natural uranium, which is comparable to the amount used in some of today's reactors. (The remaining 97% was non-fissile 238U.) Because 235U has a shorter half life than 238U, and thus decays more rapidly, the current abundance of 235U in natural uranium is about 0.7%. A natural nuclear reactor is therefore no longer possible on Earth without heavy water."
-http://en.wikipedia.org/wiki/Natural_nuclear_fission_reactor
The TMI reactor was a light water reactor so that isn't a possibility as a means of a short-lived fissioning in the natural underground uranium ore present under TMI. Is there some other way?
Marie heard something. People may not listen to her because of her manner of speech, but she heard something. What came seeping out of the ground?
Posted by: Some guy on the innernet at March 22, 2011 10:31 PMThere's a "behave" missing in that last post somewheres.....
Posted by: Aaron Datesman at March 22, 2011 10:48 PMAaron,
Yes, we seem to have engaged the same problem from different angles. My sympathies on the neon thing. For my own part, I mislabeled the units for the diffusion coefficient (should be cm^2/sec, not cm^2/gm). But the numerical value was correct, and I'm gratified to see that your value for CO2 in air (0.16 cm^2/sec) is consistent with the value obtained with the square-root-density approximation (0.17 cm^2/sec).
Although it is straightforward to calculate, I personally think that using the characteristic diffusion length can misleadingly 'compact' the actual extent of spreading.
Donald (and Aaron),
if you put the smoke inside a jar, let it reach room temperature and then opened the jar, would it really take hours for the smell to reach your nose a few feet away? My guess is no.
I also guess no, per the above. Trace amounts will arrive relatively quickly, and our olfactory senses are quite sensitive. Hint to Aaron: this has relevance to the ozone issue...
Posted by: SunMesa at March 23, 2011 12:19 AMSunMesa,
I agree about the diffusion length. To be absolutely correct, I could have written "After one hour, erf(1) (in percent) of the krypton in the balloon remains within an approximately spherical volume of radius r+480mm, where r is the radius of the balloon." However, come on - I got to drip acid on a huge balloon!
Ozone is an interesting case. Most people who have generated it (as I have, with ozone cleaners, welders, and a very large Tesla coil I built myself) think it has a smell. The internets seem not to agree. However, since I gave myself a case of ozone poisoning with the Tesla coil by failing to ventilate it properly, I come down on the side of it not having a smell. I think the smell we associate with ozone is actually some other smell produced in an ancillary way by ozone generation.
Anyway, I'm sure we'll argue about that when I post it. Maybe not tonight. I'm tired and need a drink very badly.
Posted by: Aaron Datesman at March 23, 2011 09:18 AM@Some guy -
Thanks for posting those questions, but I have no idea. Come on! I have a hard enough time figuring out how to explain ashphyxiation, heat, and a mysterious blue glow.
Seriously, that puzzles me, too. But I have no worthwhile idea.
Posted by: Aaron Datesman at March 23, 2011 09:53 AMBack when I used to hang out with physics majors, those influenced by earth science and the scope and breadth of our earth used to say that it takes five days of "something" to be in the atmosphere, and then the substance is perfectly diffused across the planet.
Although it's been some time since I have hung with these folks, if it was a true statement twelve yrs ago, it is a true statement now.
And hanging out at different blogs over the last seven days, I have encountered two people who were reminiscing abt friends they had who lived near TMI, and how they were dead of cancer within five years. Young twenty somethings, who should still be with us.
Also few are mentioning that the fuel at the number three reactor is a moxie fuel containing plutonium. This ups the ante on how serious this is.
Posted by: carol dagg at March 25, 2011 11:59 PM


